
One of the University of Waterloo’s top scientists uses neutron beams to help develop the energy storage technology needed to power electric vehicles—and to reduce the need for fossil fuels to back up wind turbines.
Source: Canadian Institute for Neutron Scattering (CINS)
Contact: webmaster@cins.ca
Image: The 31.5 MW Grand Ridge energy storage facility near Chicago. (Invenergy)
If there was a way to store the energy produced by wind turbines, then the emissions from wind power would drop dramatically…
For years, the need for better energy storage technology has been one of the foremost challenges facing the clean energy sector. Perhaps this challenge is most evident in light of electric vehicles and their energy storage limitations. Indeed, despite recent improvements, the batteries that power electric vehicles are still expensive, and their performance degrades over time.
A lesser known (but no less important) challenge is our need to store the energy that is created by renewable sources, such as wind, on the large scale required for the electricity grid. While wind turbines create a lot of clean energy, this energy generally isn’t stored; it is only available when the wind is blowing. Thus, natural gas is often required to fill the energy gap created whenever the turbines aren’t turning. For instance, in Ontario, for every kilowatt-hour produced from wind, another four kilowatt-hours of gap-filling electricity are needed from natural gas plants. As a result, today’s wind energy isn’t nearly as ‘clean’ as it could be. In fact, the need for natural gas backup means that using wind energy actually results in emissions of over 30 times more greenhouse gases than would be the case if this fossil fuel backup wasn’t required.
However, if there was a way to store the energy produced by wind turbines (and thereby eliminate the need for natural gas backups altogether), then the greenhouse gas emissions from wind power would drop dramatically, making its lifecycle emissions just about as low as those of the cleanest energy sources out there—namely hydroelectric, tidal, and nuclear power.
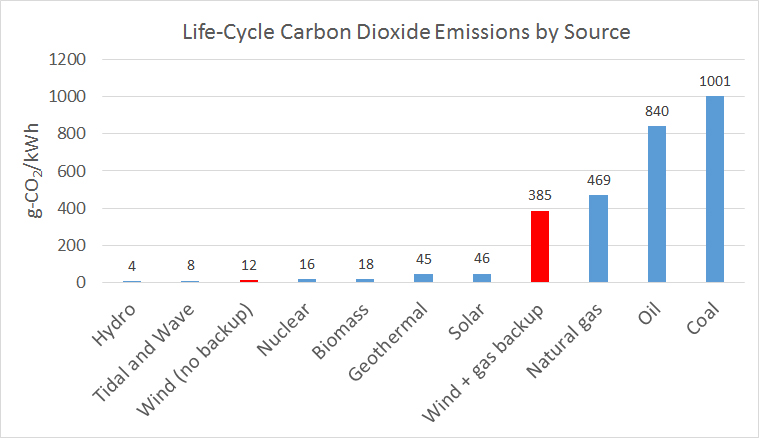
Lifecycle carbon dioxide emissions from various energy sources per kilowatt-hour (kWh) of electricity produced.[1]

Linda Nazar (left) receives her appointment as an Officer of the Order of Canada, one of Canada’s highest honours, from Governor General David Johnston.
Notably, this cost could be significantly reduced by increasing the amount of energy that can be stored in the same amount of material (i.e., the energy density). Unfortunately, increasing a battery’s energy density is no easy feat because today’s battery technologies are already pushing the limits of the materials’ capabilities.
Professor Linda Nazar of the University of Waterloo is a world leader among scientists tackling this challenge. Her work has been cited by other scientists over 20,000 times, making her one of the world’s most respected materials researchers. Nazar’s accomplishments include the demonstration of lithium-sulfur battery technology, as well as numerous influential discoveries in lithium and sodium battery systems. Her progress has been accelerated by partnering with industrial leaders in the field such as General Motors (GM) Canada, Hydro-Québec, and BASF, a multi-national chemical manufacturer. In recognition of her achievements, Nazar was inducted as an Officer of the Order of Canada in 2015.
Increasing a battery’s energy density is no easy feat because today’s battery technologies are already pushing the limits of the materials’ capabilities.
Nazar’s goal is to develop better materials for batteries with higher energy density. Batteries themselves are relatively simple devices consisting of two electrodes, typically metals, that release or absorb charged particles (e.g., lithium ions in the case of lithium-ion batteries). A liquid or solid medium (the electrolyte) allows the charged particles to move between the electrodes, and this movement creates electricity.
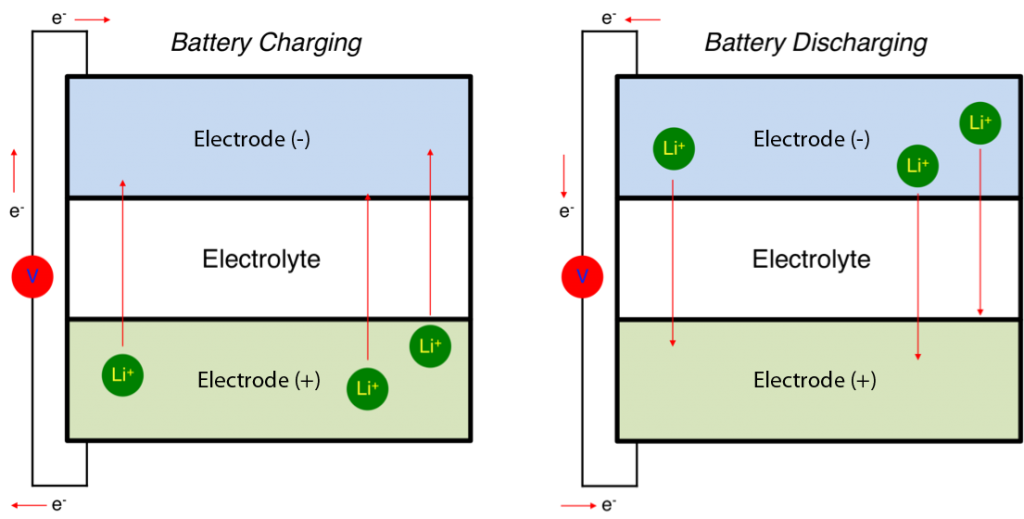
Diagram of a lithium-ion battery. The movement of positive ions (green, Li+) through a medium (the electrolyte) is offset by the movement of negative electrons (e–) through a circuit (with voltage represented by the red V), thereby creating an electrical current. (Image: Battery Bro)
Nazar’s research team employs the best experimental techniques, including the use of neutron beams, to study electrode materials. “Neutrons are indispensable for the structural analysis of battery electrode materials that contain lithium, and are also very important for ones containing sodium,” explains Nazar. That’s because lithium and sodium atoms are relatively small in size, atomically speaking. As such, when they are surrounded by much larger atoms typically found in electrodes, they become nearly invisible to x‑rays—but they can still be detected by neutrons.
One line of Nazar’s research is focused on finding better materials for the electrodes in lithium-ion batteries, which are used in electric vehicles and many electronic devices. One candidate electrode material that her team has investigated with the support of GM Canada is lithium (Li) iron (Fe) fluorosulphate (SO4F), or LiFeSO4F. In 2009, this new material was reported to have auspicious properties that could lead to increasing the energy density and lowering the cost of lithium-ion batteries. The discovery of LiFeSO4F’s potential sparked a flurry of scientific activity to characterize its behaviour and find effective means to produce it.
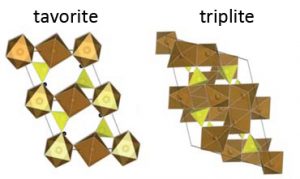
Illustration of the crystal structures of the two polymorphs of LiFeSO4F.
Research showed that LiFeSO4F can exist in at least two different forms, known as ‘polymorphs,’ in which the atoms arrange in distinct patterns (i.e., ‘crystal structures’). A key question that Nazar set out to answer was how the different versions of LiFeSO4F’s crystal structure affected the material’s electrochemical properties—namely, its ability to store lithium ions.
Nazar’s team first had to find a way to make pure samples of the two polymorphs of LiFeSO4F separately. To do so, the team adapted a ‘solvothermal’ production method that it had used previously to make similar materials by essentially ‘cooking’ a ‘soup’ of constituent elements using a science-grade pressure cooker.
In addition to x‑rays, Nazar’s team used neutron beams at Oak Ridge National Laboratory (ORNL) in the U.S. to not only verify that they had successfully made the two polymorphs of LiFeSO4F, but also to fully solve the crystal structures for each. The researchers were able to confirm that one of the LiFeSO4F polymorphs had what’s known as a ‘triplite’ crystal structure, while the other had a ‘tavorite’ structure. They then tested how these two polymorphs performed as electrodes in a battery-like environment.
The team’s findings, published in 2012, showed that of the two polymorphs of LiFeSO4F, the triplite version absorbed lithium ions at higher voltages—which, notably, results in higher battery energy density. On the other hand, the triplite also had slower charging and discharging times than the tavorite. Knowledge of the crystal structures provided an explanation: while the tavorite’s atomic arrangement provides open channels for the infusion of lithium ions, the triplite arrangement is prone to blockages that force the ions to take a slower zig-zag route through the material.
In follow-up research published in 2013, Nazar’s team showed that the performance of the triplite could be enhanced twofold if they modified their solvothermal ‘cooking’ process to use microwaves (i.e., electromagnetic radiation) as the heat source. Combined results from x‑rays and neutron beam experiments showed that, while the microwaved triplite’s basic structure remained the same, it also contained more defects at the nanoscale. Notably, in this case, the defects actually helped the triplite to keep its channels open, resulting in the more rapid infusion of lithium ions (i.e., faster charging and discharging times) at the same high voltages.
The impact of this research is twofold. First, it provides clear explanations as to why LiFeSO4F performs as it does. Although interest in this material for large-scale energy storage has cooled in the intervening years, Nazar believes that materials like LiFeSO4F will recapture research interest in the future because they hold promise for applications where safety is critical. Second, it clearly shows the usefulness of their ‘cooking’ methods, not only for making electrode materials for battery research, but also for adjusting the performance of these materials. Nazar’s team has continued to successfully use their methods to tune the performance of other electrode materials, most recently publishing a study on this topic in Nature Energy in 2016.
Neutrons are indispensable for the structural analysis of battery electrode materials that contain lithium, and are also very important for ones containing sodium. … There is a lot of competition for time on foreign neutron beamlines to do research. The demand from the science community is much greater than the supply.
In a second line of research, Nazar is exploring electrode materials for sodium‑ion batteries. Because sodium is much more abundant and less expensive than lithium, it could be better suited for the large-scale energy storage systems needed for the electricity grid.
One electrode material that has been a focus of attention recently is sodium (Na) manganese (Mn) oxide (O2), or Na0.7MnO2, due to its low cost and environmental friendliness. Although researchers didn’t agree on some particulars of its structure, previous studies had shown that beneficial effects could be achieved by replacing some of Na0.7MnO2’s manganese with other metals such as iron or nickel. However, after replacing half of its manganese with iron (Fe) to produce Na0.67[Mn0.5Fe0.5]O2, Nazar’s team observed that the material’s performance degraded when exposed to air.
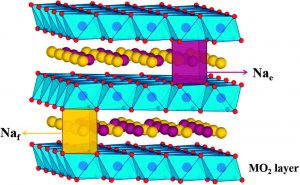
Illustration of the crystal structure of a sodium manganese iron oxide, in which the metals manganese (pink) and iron (blue) are randomly distributed within the metal oxide layers. The sodium ions lie between these layers in two configurations (illustrated by orange and green spheres) that differ slightly in their distance from the metal atoms.
The researchers set out to understand why such degradation occurred and to test ways of tackling this problem. Using samples of Na0.67[Mn0.5Fe0.5]O2 that were carefully protected from air, Nazar’s team once again used x‑rays and neutron beams at ORNL in the U.S. to investigate the material’s behaviour at the atomic level. Their findings provided concrete insights into how air causes the material to degrade. Their results also showed that previous studies on iron-containing manganese oxides like Na0.67[Mn0.5Fe0.5]O2 had underestimated how reactive these materials are with air, which provided an explanation for the conflicting observations about the structure of these materials in prior studies.
Even more importantly, Nazar’s team was able to show that the material (so long as it was protected from air) did indeed have an excellent ability to absorb and release sodium ions in battery-like conditions. In findings published in March 2015, the team’s combined x‑ray and neutron data explained this superior performance by shedding light on the material’s crystal structure. The sodium atoms were sandwiched between metal oxide layers. These layers were composed of manganese and iron atoms surrounded by oxygen atoms. Generally speaking, the metals in metal oxide layers such as these could be arranged in an orderly and repeating pattern. In Na0.67[Mn0.5Fe0.5]O2, however, the manganese and iron atoms were randomly distributed, corresponding to a disordered configuration. In this case, the disordered configuration turned out to be beneficial, as it helped the material to resist detrimental changes to its structure during charging that have been observed in closely related materials, such as Na0.67[Mn0.67Fe0.33]O2.
Perhaps the most important finding to emerge from Nazar’s investigations of sodium manganese iron oxide relates to the effects of doping this material with nickel (Ni). Indeed, adding nickel did a lot to increase the material’s stability in air, leading the researchers to explore the new nickel-doped material, Na0.67[Mn0.65Ni0.15Fe0.2]O2, in a further study published in July 2015. When used as an electrode, the nickel-doped material delivered 25 percent more energy and maintained its performance better over time than the undoped material.
Nazar’s team has since published additional research further improving the performance of these materials.
To figure out why, the team once again used x‑rays as well as neutron beams at ORNL to solve the structure of the nickel-doped material. This better understanding of the new material’s atomic makeup led the researchers to conclude that nickel-doped Na0.7MnO2 is in fact a highly promising material for future development because there is likely a lot of potential to optimize its performance and bring it closer in line with market applications.
“Variations of these sodium manganese oxide materials are still hot topics in the scientific community,” says Nazar. Her team has continued to build on the insights gained from its neutron beam experiments, and has since published additional papers that show the improved performance of these materials when doped with lithium.
Although neutrons have played an important role in her research, Nazar notes that “There is a lot of competition for time on foreign neutron beamlines to do research.” In her view, “The demand from the science community is much greater than the supply”—a fact that makes it challenging for even the world’s best researchers to access the tools they need to make clean vehicles and renewable energy an everyday reality.
[1] Intergovernmental Panel on Climate Change (2011). “Special Report on Renewable Energy Sources and Climate Change Mitigation.” Data on natural gas backup for wind turbines: Hatch (2014). “Lifecycle Assessment Literature Review of Nuclear, Wind and Natural Gas Power Generation.”
