
Scientists regularly access neutron beams to gain fundamental insights into the inner workings of new materials, including some with potential for clean energy applications.
Source: Canadian Neutron Beam Centre (CNBC)
Contact: cnbc@cnl.ca
Image: ‘Blue sky research’ is another name for basic research. (Credit: HolHom)
Canada participates in Mission Innovation, a multinational commitment associated with the 2015 Paris Agreement on climate change. Members of Mission Innovation plan to double investments in clean energy research and development within five years. Such research includes studies that directly contribute to the advancement of specific energy technologies like wind turbines, nuclear power, and hydrogen-powered vehicles. (For a description of how neutron beams contribute to some of this research, see other articles on clean energy.)
Mission Innovation also includes ‘basic energy research,’ which aims to lay general scientific foundations upon which clean energy advances can be based in the future. Basic research is particularly important when it comes to the new materials that researchers around the world are producing on a regular basis. The scientific community must gain a fundamental understanding of these materials’ underlying properties before their potential for applications, whether for energy or otherwise, can be evaluated properly. During these basic investigations, scientists use an array of cutting-edge tools, including neutron beams, to learn all they can about each new material and about how it might one day be used to improve our lives.
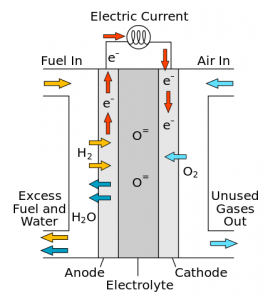
A solid oxide fuel cell, which makes electricity—moving electrons (e-)—by combining hydrogen (H2) with oxygen (O2). It is clean technology because water (H2O) is the only by-product. (Image: Sakurambo)
Some of these new materials are members of a family known as ‘perovskites,’ which are often used in clean energy applications. The name refers to the periodic arrangement of the atoms that defines the perovskite structure. Their structure is one factor that gives perovskites excellent properties for converting waste heat into useful energy, for mediating chemical reactions, and for storing electricity. For that reason, perovskites are often used in fuel cells, which are devices that create electricity from a chemical reaction, often producing water as the by-product.
Professors Mario Bieringer from the University of Manitoba and John Greedan from McMaster University are two researchers who are conducting basic research on new materials in the perovskite class. Both researchers use neutron beams at the Canadian Neutron Beam Centre (CNBC), among other tools, to carry out their investigations, as neutron diffraction is a leading way to ‘solve the structure’ (i.e., precisely determine the arrangement of atoms) of a material.
Bieringer’s research team has been studying barium cerate (BaCeO3), a perovskite compound that, when doped, is suitable for conducting hydrogen ions in fuel cells to produce clean electricity. “Understanding the details of these materials’ structures and how stable they are at high temperatures is crucial for optimizing the performance of solid oxide fuel cell materials because of their high operating temperature, which can be up to 1000 °C,” explains Bieringer.
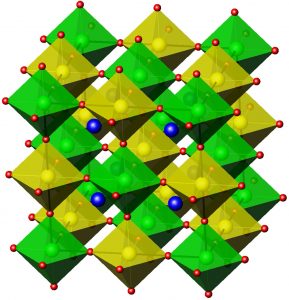
The double perovskite structure of Ba2CePtO6. Gold and green octahedra are CeO6 and PtO6, respectively. Red and blue spheres are oxygen and barium, respectively. (Image: American Chemical Society)
In 2010, while using x‑rays to study barium cerate doped with indium (In), Bieringer’s research team observed an unexpected chemical change in the material over a narrow range of high temperatures. Two other research groups had already observed that barium cerate could absorb some atoms from a container made of platinum (Pt), an element that is frequently used as a catalyst in fuel cells. However, very little data was available on the new material that was being formed—namely, Ba2CePtO6—or how it behaved under various conditions.
To help fill this knowledge gap, Bieringer’s team used neutron beams at the CNBC as well as x‑rays to more thoroughly investigate barium cerate’s structure under the influence of two different dopants. They particularly wanted to determine the impact that these dopants had on the formation of Ba2CePtO6.
The results, published in 2014, demonstrated that the arrangement of atoms in Ba2CePtO6 forms a pattern known as a ‘double perovskite.’ They further showed that some Ba2CePtO6 formed regardless of which dopant was used, or how much dopant was used, or even if no dopant was used at all. Insights were also gained into the compound’s chemical pathway. Specifically, the results suggested that Ba2CePtO6 could be an intermediary state in which the platinum helps form the perovskite structure in the doped barium cerate, which is desirable for fuel cell applications.
Like Bieringer, Greedan has also studied the structure of numerous perovskites. One of these materials, SrFeO2F, is a type of perovskite known as a ‘cubic oxyfluoride.’ Oxyfluorides can be used to produce luminescent glass lenses, which have the potential to make electricity-conserving LED lights simpler and less expensive to manufacture while offering greater control over the light’s colour than current LEDs do.
Laying the foundation to realize the potential of oxyfluorides requires scientists to first characterize their structure. That entails solving not only their atomic structure, but also their magnetic structure (i.e., the directional pattern of the tiny magnetic fields associated with the material’s electrons), as this pattern plays a vital role in determining the magnetic and electric properties of the material.
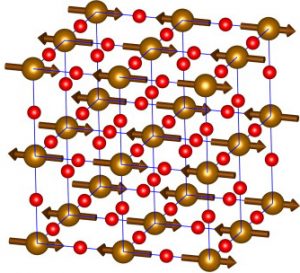
The magnetic and atomic structures of the perovskite SrFeO2F, a cubic oxyfluoride. The magnetic structure is represented by arrows, while the atomic structure is shown using red (Fe) and gold (O2F) spheres. (Image: Elsevier Inc.)
Prompted by a set of open questions published by other researchers about the structure of SrFeO2F, Greedan and his team set out to learn more about this compound. They conducted their systematic exploration of the material using neutrons at the CNBC, as neutron diffraction is the best way to study a material’s magnetic structure.
Accordingly, after taking a series of measurements at temperatures ranging from –270 °C to 500 °C, Greedan’s team was able to solve the material’s magnetic structure while also helping to resolve some of the outstanding questions about its atomic structure. They published an article on this structure in 2014, with another in 2015 noting further refinements. Their exploratory study also enabled them to better understand how the atomic and magnetic structures work together to produce observable properties.
Although there’s no guarantee that SrFeO2F or Ba2CePtO6 will play a role in the clean technologies of the future, the pioneering studies conducted by Bieringer and Greedan gathered fundamental data about these new materials. Without basic energy research investigations such as these, the scientific community would lack a solid foundation of knowledge on which to base future research and development. Indeed, today’s fundamental research will pave the way for tomorrow’s clean energy technologies—as well as for other applications we may not even be able to imagine yet.
