American physicists accessed the CNBC to map the inner atomic workings of a compound within a mysterious class of materials known as spin-orbit Mott insulators.
Source: Canadian Neutron Beam Centre (CNBC), adapted from ScienceDaily.com
Contact: cnbc@cnl.ca
Theorists have predicted the emergence of new properties in spin-orbit Mott insulators, at points just beyond the insulating state, when electronic manipulation can transform these compounds into conducting metals. A better understanding of electrons near this transition could allow these new materials to pave the way to discoveries in superconductivity, new topological phases of matter and new forms of unusual magnetism. What scientists lacked until now was experimental evidence that reveals the microscopic mechanisms that actually drive one of these spin-orbit Mott insulators to become a metal.
Spin-orbit Mott insulators have complex electronic properties and are characterized by a delicate interplay between repulsive action and the coupling between electron spin and orbital motion. Specifically, the repulsive interaction between electrons tends to drive the electrons to a standstill. This tendency is bolstered by the lowering of the electron’s energy via a strong interaction between the electron’s magnetic field and its orbital motion around the nucleus.
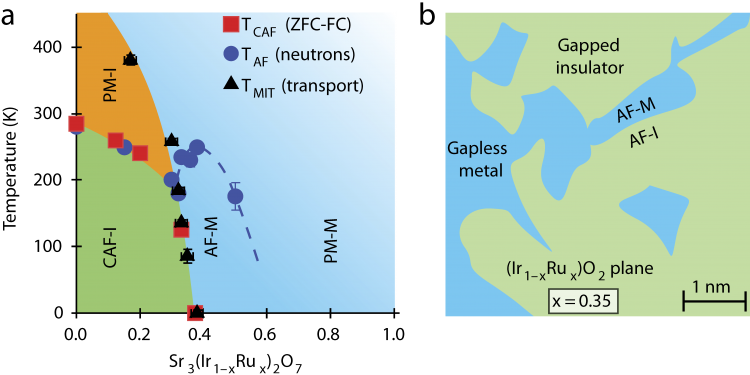
A team of physicists led by Professor Stephen D. Wilson at Boston College (now at the University of California Santa Barbara) manipulated a compound of strontium, iridium and oxygen (Sr3Ir207), successfully driving it into the metallic regime by replacing 40 percent of the iridium ions with ruthenium, thereby creating a metal alloy, Sr3(Ir1‑xRux)2O7.

Boston College graduate student Chetan Dhital (pictured) gained hands-on experience at the N5 beamline while working under CNBC Scientist Dr. Zahra Yamani to perform the experiments. Dr. Yamani also led the project to upgrade the detector’s shielding
The team, which included researchers from Oak Ridge National Laboratory, the NIST Center for Neutron Research and the Canadian Neutron Beam Centre, obtained a unique view into the workings of these insulators by mapping this previously uncharted transformation as it took place. The team created this map by combining scanning tunnelling microscopy and other techniques with neutron scattering to determine the electronic phase diagram of Sr3(Ir1‑xRux)2O7 in detail.
Neutron scattering experiments were performed at the CNBC’s N5 triple-axis spectrometer to study how the antiferromagnetic order was affected by temperature and the amount of ruthenium ions. The N5 beamline was newly upgraded with improved shielding to increase the instrument’s sensitivity. This upgrade was critical to the experiment because large single crystals of Sr3(Ir1‑xRux)2O7 are difficult to grow. In fact, the crystals were tiny, with dimensions of only about 2 x 2 x 0.1 mm and weighing only a few milligrams, and yet the upgraded instrument was sensitive enough to collect the required data.
Combining the data from all the techniques used, the team inferred that the ruthenium effectively created features within the compound that resembled minute metallic puddles. As the amount of additional ruthenium was increased, the puddles began to ‘percolate,’ coalescing to form a metal across which charges freely flowed.
In Prof. Wilson’s words, “The addition of ruthenium introduces charge carriers, but at a low ratio of ruthenium to iridium they simply stay put in these little metallic puddles, which are symptoms of strongly correlated electrons. These electrons are stable and wouldn’t move much. But when we stepped up the disruption by increasing the amount of ruthenium, the puddles moved together and achieved a metallic state.”
Furthermore, according to Prof. Wilson, “The behavior in this particular compound parallels what researchers have seen in Mott insulators that play host to such phenomena as high temperature superconductivity. By pinpointing exactly where this transformation takes place, the team’s findings should help to lay the groundwork in the scientific search for new electronic phases within spin-orbit Mott insulators.”
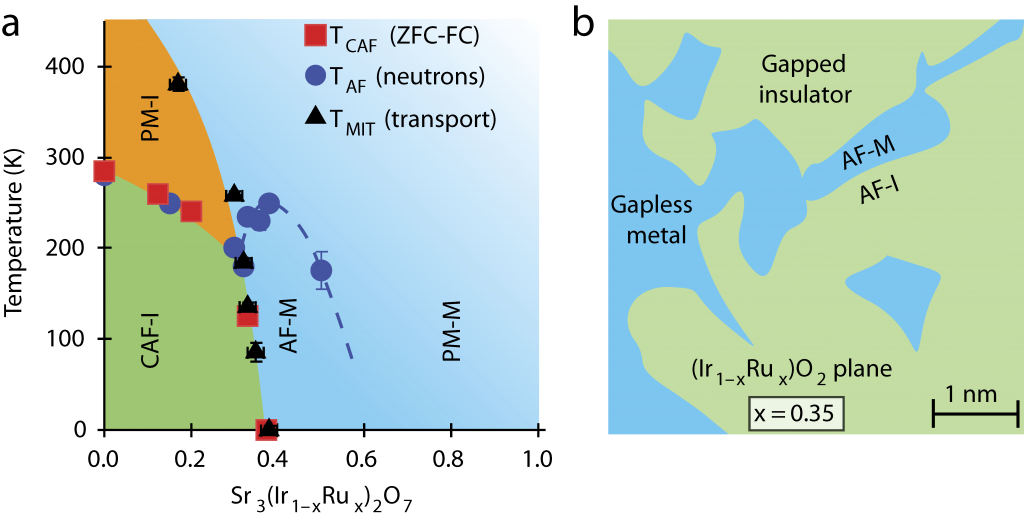
(a) Electronic phase diagram of Sr3(Ir1 xRux)2O7 as a function of ruthenium (Ru) concentration and temperature. (b) Illustration of the basal plane showing phase-separated metallic puddles near the percolative threshold, which nucleate within the spin-orbit Mott insulating background of Sr3Ir2O7.
